Nick Gulliver
Nick Gulliver is chief nuclear medicine technologist and an advanced practitioner at NHS Greater Glasgow & Clyde
Luisa Roldao Pereira
Luisa completed a BSc in Nuclear Medicine and the MSc in Advanced Clinical Practice (ACP) at the University of Brighton. Her career included roles such as departmental superintendent, RPS, ACP in NM therapies, vice-chair of the EANM Technologists Committee and lead for advanced practice in her NHS trust.
Nick Gulliver
Nick Gulliver is chief nuclear medicine technologist and an advanced practitioner at NHS Greater Glasgow & Clyde
Luisa Roldao Pereira
Luisa completed a BSc in Nuclear Medicine and the MSc in Advanced Clinical Practice (ACP) at the University of Brighton. Her career included roles such as departmental superintendent, RPS, ACP in NM therapies, vice-chair of the EANM Technologists Committee and lead for advanced practice in her NHS trust.
IMolecular imaging and nuclear medicine have undergone significant advancements in recent years, particularly in the development of novel radiopharmaceuticals and in advances to PET and SPECT imaging technologies. These advances have not only expanded the possibilities of diagnostic imaging but also opened new avenues in therapeutic applications. Notable innovations include radiopharmaceuticals like 177Lu-PSMA (Lutetium-177 Prostate-Specific Membrane Antigen) and 68Ga-FAPI (Gallium-68 Fibroblast Activation Protein Inhibitor), as well as targeted alpha-emitting therapies (TAT). These developments, coupled with new artificial intelligence (AI) applications and new imaging hardware Long Axis Field-of-View PET-CT (LAFOV), are transforming nuclear medicine, enhancing diagnostic accuracy, and improving patient outcomes. This article explores some of these recent advances and the emerging technologies shaping the field.
68Ga-FAPI PET for oncology imaging
Positron emission tomography (PET) is a commonly used imaging technique in clinical oncology. Among PET radiotracers, 18F-fluorodeoxyglucose (FDG) has been especially valuable for staging, restaging, detecting recurrences, and predicting prognosis across various cancers. While 18F-FDG remains an essential radiotracer and will remain the “workhorse” tracer for PET for many years to come, there has been growing research into other radiopharmaceuticals to enhance prediction and monitoring of therapeutic responses, particularly with the advancement of targeted therapies.
68Ga-FAPI has emerged as a versatile PET imaging agent with broad applications across multiple cancer types (1). FAPI targets fibroblast activation protein (FAP), which is expressed in cancer-associated fibroblasts of the tumour microenvironment. This unique targeting capability allows 68Ga-FAPI to visualise not only the primary tumour but also its stromal components, providing a more comprehensive picture of tumour biology.
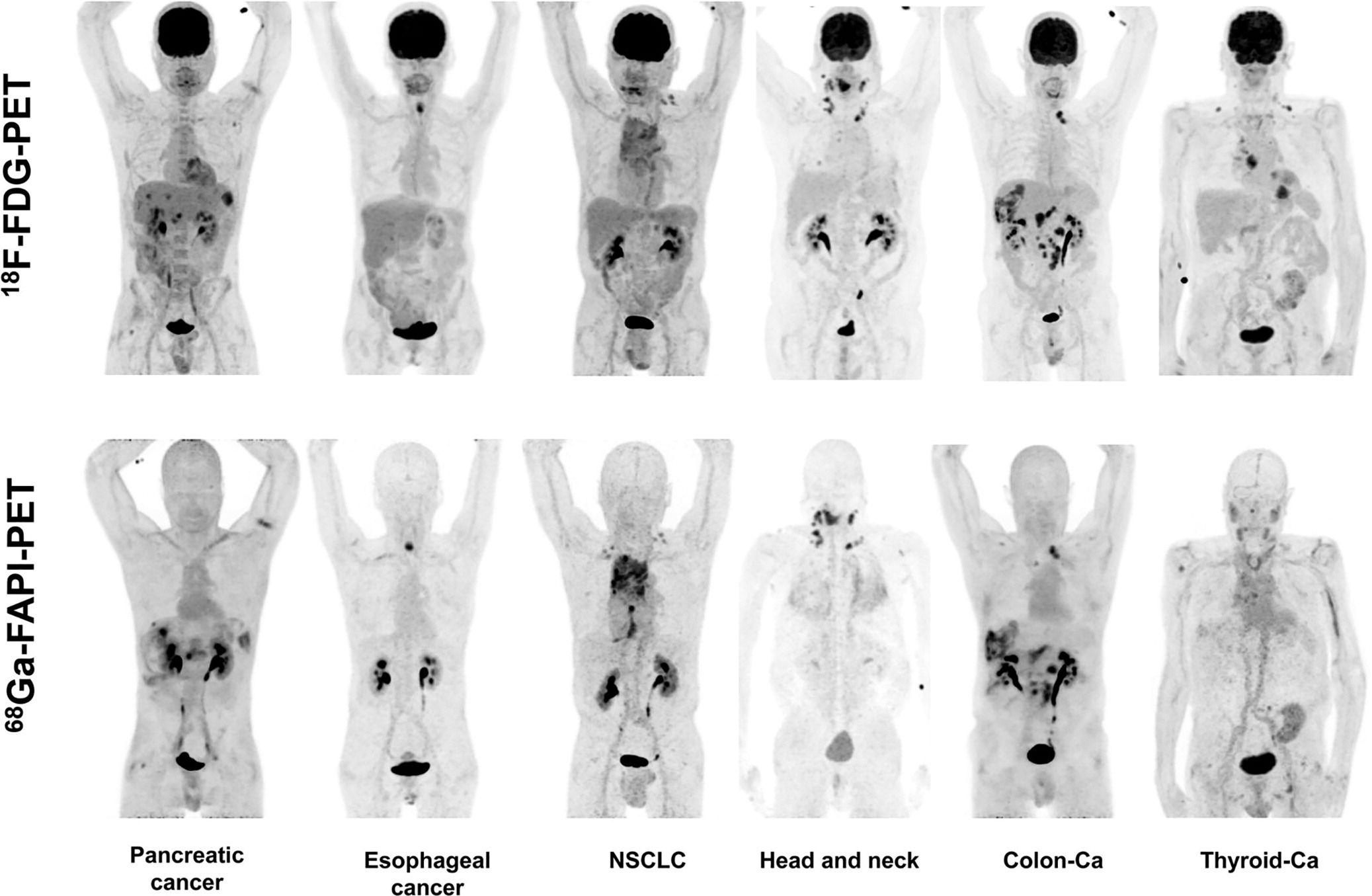
Compared to 18F-FDG, 68Ga-FAPI offers high uptake in a variety of tumours, including breast, pancreatic, and colorectal cancers, among others. It exhibits low background uptake in normal tissues, resulting in high-contrast images that enhance diagnostic accuracy (Figure 1) (2). 68Ga-FAPI also enables faster imaging protocols compared to 18F-FDG with minimal delay between injection and scanning, which is particularly beneficial for clinical workflow and patient comfort.
Figure 1
The rapid clearance of 68Ga-FAPI from non-target tissues means it provides high-contrast images, but this also limits its utility for therapeutic applications (3). Research is ongoing to develop versatile versions of FAPI, pairing it with therapeutic isotopes that can deliver a cytotoxic dose to the tumour stroma, potentially turning FAPI into a dual-purpose agent for both imaging and therapy (successfully delivering the concept of “theranostics”) (4).
Immuno-PET agents for imaging of immune checkpoint inhibitor therapy
Monoclonal antibodies (mAbs) targeting immune regulatory checkpoints play a major role in improving anti-cancer treatments in patients. An inherent limitation of using mAbs as a molecular imaging probe is its pharmacokinetics which result in a prolonged circulating half-life and slow clearance from the body, which leads to undesirable target to background ratios during imaging. Radioisotopes with short half-lives, such as 18F (t1/2 = 110 min), 11C (t1/2 = 20 min) and 13N (t1/2 = 10 min), which are common in clinical practice, have the advantage of low radiation exposure but are not optimal for imaging mAbs (5). In immuno-PET, radiolabelling with positron-emitting radionuclide Zirconium-89 (t1/2 = 78.4 hours) allows non-invasive tracking of mAbs as well as longitudinal studies.
89Zr-immuno-PET enables personalised dosing and monitoring of therapeutic antibodies, particularly valuable in immuno-oncology where predicting response to immune checkpoint inhibitors remains challenging.
The continuing expansion of 89Zr-based PET imaging for a range of immunotherapies will likely refine patient selection and optimise dosing for targeted immunotherapy, contributing to the broader field of personalised cancer therapy (2).
Game-changing imaging technologies: Long Axis FOV (Total Body) PET-CT
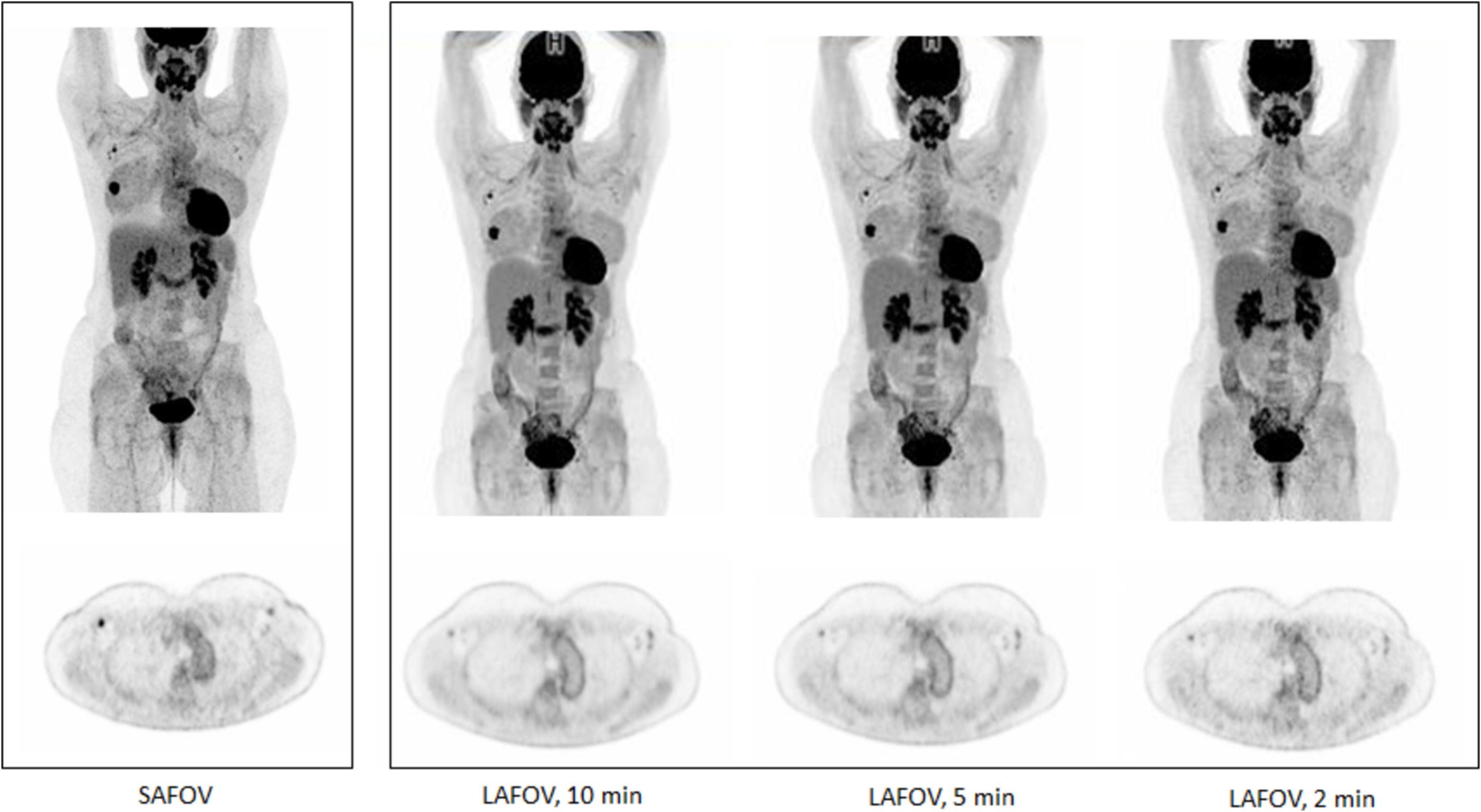
Long Axis Field-of-View (LAFOV) PET-CT systems, sometimes referred to as “total-body” PET-CT scanners, are revolutionising the way molecular imaging is conducted (6). Unlike traditional short axis FOV (SAFOV) PET-CT scanners that have a limited field-of-view (FOV) of 20-30cm (therefore requiring sequential imaging acquisition over the length of the body), LAFOV PET-CT scanners cover the entire body in a single scan. This allows for simultaneous imaging of multiple organs, yielding comprehensive insights into whole-body pathology in a single acquisition.
LAFOV PET-CT systems incorporate advanced detector technology and an extended axial FOV, up to a maximum currently of 194cm, which enables whole-body imaging without requiring patient repositioning. These systems also have substantially higher sensitivity which means the detectors measure a higher count rate of coincidence photons per unit activity inside the PET detector, thus allowing for lower radiotracer doses and reduced scan times. For example, a total-body scan that would take 20–30 minutes on a SAFOV PET-CT scanner can now be completed in under two minutes with LAFOV systems, reducing patient discomfort, movement artefacts, and improving throughput (Figure 2) (7).
Figure 2
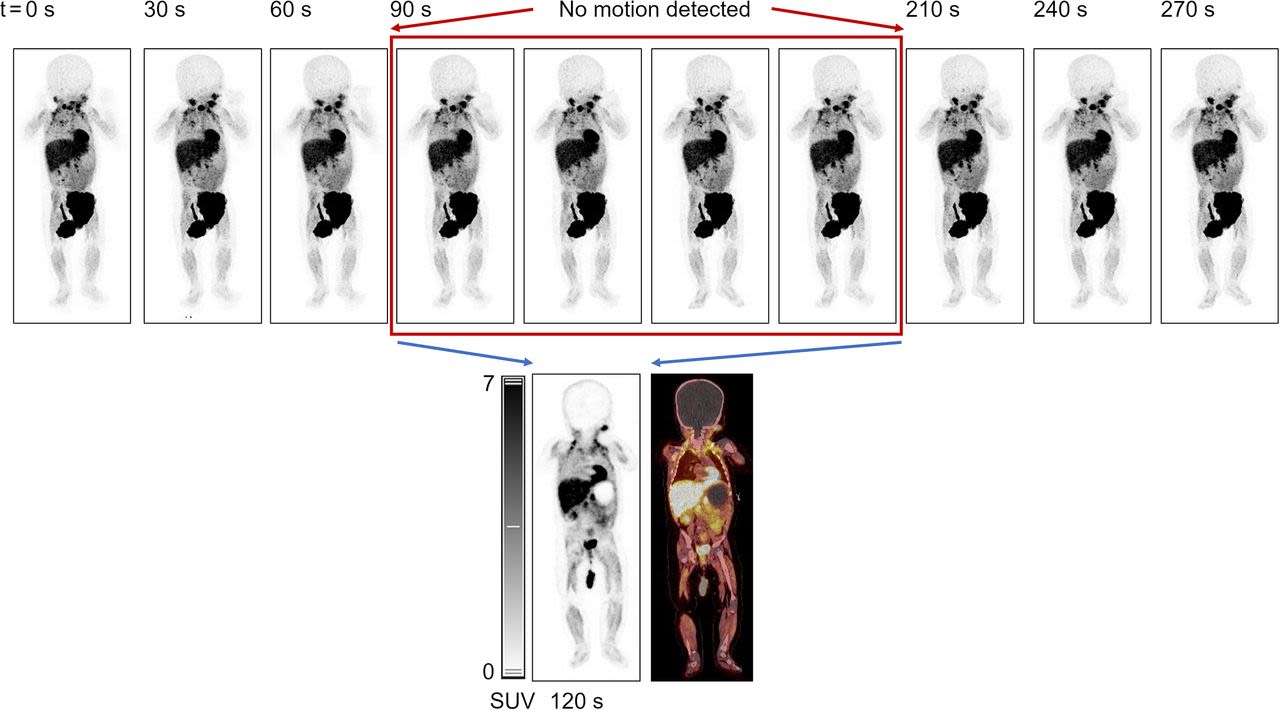
In paediatric imaging, the challenges of keeping the child calm and laying still is essential for achieving diagnostic image quality by reducing motion artefacts. This increased speed of image acquisition is therefore driving the ability to scan paediatric patients without any need for sedation (Figure 3) (8). Dose optimisation is of paramount concern in paediatric imaging due to the heightened sensitivity of developing tissues to ionising radiation and the potential long-term risks. Some initial studies with paediatric patients scanned with LAFOV PET-CT systems have demonstrated that it is possible to reduce injected dose to up to 1/10th of the usually recommended dosage allowing for 18F-FDG LAFOV PET-CT scanning with dose exposures below 1 mSv without significant degradation of image quality and diagnostic accuracy.
Figure 3
This ability to achieve diagnostic quality images at very low injected doses also opens the door to imaging other patient cohorts and populations previously not imaged with SAFOV PET-CT. Some initial studies have demonstrated how a 10% of normal injected dose coupled with the increased sensitivity of LAFOV PET-CT systems can reduce the radiation dose to the foetus to around 0.1mGy (9).
The high sensitivity of LAFOV PET-CT enables dynamic imaging and kinetic modelling, which are not feasible with SAFOV PET-CT scanners. The extraction of high-quality lesion and arterial blood time activity curves from dynamic data allows for parametric imaging of biological parameters other than the conventionally used standardised uptake value (SUV). This feature is particularly useful for real-time tracking of radiopharmaceuticals in the body, studying disease kinetics, and measuring pharmacodynamics in response to therapy. Furthermore, the whole-body nature of LAFOV PET-CT is highly beneficial for conditions that require systemic assessment, such as metastatic cancers and inflammatory diseases. Recent studies have also demonstrated that the increased sensitivity of LAFOV PET detectors can be exploited to detect the intrinsic background radiation that is present in lutetium orthosilicate (LSO) scintillators in some PET systems, and utilise this as a transmission source for attenuation correction instead of low dose CT.
While LAFOV PET-CT offers significant benefits, challenges remain in its widespread adoption, primarily due to the high costs associated with the equipment and installation. Current research is focused on developing more cost-effective versions of these systems, as well as optimising imaging protocols to leverage the technology's full potential. Additionally, data generated from LAFOV PET-CT is extensive, necessitating advanced computational methods for efficient image processing and analysis. (7)
Integrative approaches: theranostics and personalised medicine in nuclear medicine
Theranostics—integrating therapeutic and diagnostic functions within a single radiopharmaceutical platform—is a major trend in nuclear medicine, particularly with radiopharmaceuticals like PSMA and FAPI agents. This approach aligns with the improvement towards personalised medicine by enabling clinicians to tailor treatments based on the molecular characteristics of an individual patient’s disease.
PSMA-based agents, such as 68Ga-PSMA for imaging and 177Lu-PSMA or Actinium-225 (225Ac) PSMA for therapy in men with prostate cancer, are examples of successful theranostic pairs that have already shown significant clinical impact (10,11). Similarly, 68Ga-FAPI holds potential for theranostic applications when paired with therapeutic isotopes, targeting the tumour stroma rather than the cancer cells directly. This approach is promising for tumours with high stromal activity, which are often resistant to conventional therapies (12).
The future of theranostics lies in expanding the library of theranostic pairs and developing personalised treatment algorithms. Machine learning and artificial intelligence are expected to play critical roles in optimising treatment protocols based on patient-specific imaging and treatment response data (13).
Advances in therapeutic radiopharmaceuticals
An increasing number of therapeutic radiopharmaceuticals in recent years have shown promising efficacy, including 177Lu-PSMA, 225Ac-PSMA, Terbium-161 (161Tb)-DOTATATE, and Copper-64 Dichloride (64CuCl2). Both 177Lu-PSMA and 225Ac-PSMA are radiolabelled compounds that target PSMA, a transmembrane protein that is highly expressed on the surface of prostate cancer cells. The therapeutic use of 177Lu, a beta-emitting radionuclide, allows for the targeted delivery of cytotoxic radiation to PSMA-expressing tumours, sparing surrounding healthy tissues. Reports on the use of 177Lu-PSMA have demonstrated improved survival outcomes and reduced disease progression in patients with metastatic castration-resistant prostate cancer (mCRPC), a disease stage often resistant to conventional treatments (14).
While PSMA is gradually establishing itself as a viable option for patients with mCRPC across the world, the groundbreaking factor may come from considering its benefits at earlier stages of the pathway, for instance post radical prostatectomy when PSA is slowly rising again, or in metastatic hormone-sensitive prostate cancer cases.
In contrast to beta-emitting radionuclides such as 177Lu, 225Ac is a radioisotope which emits high-energy alpha particles, which present a much higher linear energy transfer (LET) than beta particles. This translates into a more localised and potent cytotoxic action on cancer cells. Alpha particles induce double-strand breaks in DNA, which are difficult for tumour cells to repair, resulting in tumour cell death even in cases where beta-emitters may be less effective (15). Recent studies have highlighted the possibility of combined therapies, some with non-radiopharmaceutical options or using both alpha and beta emitters (177Lu/225Ac), either simultaneously or sequentially (16). Naturally, caution must be observed to guarantee that the safety profile is adequately tackled. Though 225Ac-PSMA is still in its infancy, preliminary results have spurred interest in expanding alpha-particle-based theranostics as the next-generation of therapies for numerous malignancies (17).
Another “rising-star” radiopharmaceutical is 161Tb-DOTATATE, for the treatment of neuroendocrine tumours (NETs) which express somatostatin receptors (18). This follows from 177Lu-DOTATATE, already approved for unresectable or metastatic neuroendocrine tumours in adults. Analogously, 161Tb is a beta-emitting radionuclide; but its key differentiator is its half-life (6.9 days), which is well-suited for targeted therapies, offering a balance between precise radiation delivery and minimal damage to adjacent healthy tissues. In addition to the beta emission, 161Tb not only emits conversion and Auger electrons which may cause an increased therapeutic efficacy compared with 177Lu, but also gamma radiation which can be detected and imaged using widely available SPECT (Single-Photon Emission Computed Tomography) capabilities of most gamma cameras, subsequently allowing for assessment of lesion avidity and therapeutic dosimetry (19).
The multifaceted 64CuCl2 is expected to be of use to a range of cancers, such as for prostate, bladder, and glioblastoma multiforme (GBM). As a beta-emitting radionuclide (both β+ and β- particles) with a half-life of 12.7h, this is another radiopharmaceutical befitted for treatment and real-time visualisation, showing characteristic high-resolution PET imaging. Owing to its specific affinity for androgen receptor, overexpressed in many prostate cancers, along with its high serum stability, and the fact that copper ions are mostly eliminated hepatically and not via urinary tract, 64CuCl2 is likely to grant an accurate assessment of the pelvic region and inherently the prostate bed, with quick visualization of pelvic lesions. It may however have some limitations in detecting liver metastases.
Additionally, the application of 64CuCl2 in radioimmunotherapy is being investigated, considering its use with the mAb trastuzumab for treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer (20). Of note is the uptake of 64CuCl2 specifically in cells with expression of copper transporter CTR1. It has been reported that low expression of CTR1 can work as a surrogate for the uptake of platinum-based chemotherapy. As such, if low uptake is demonstrated in 64CuCl2 PET imaging, then this may be used as a predictor of poor clinical outcome when using this specific chemotherapy type (e.g. in patients diagnosed with non-small cell lung cancer). Importantly, 64Cu-PET-CT could be used to avoid the use of ineffective platinum-based chemotherapy, hence affecting patient selection and limiting it to patients who may actually benefit from it (21).
Other isotopes that are generating considerable interest for potential use in Targeted Alpha Therapy (TAT) include Astatine-211 (t1/2 = 7.2h) and Lead-212 (t1/2 = 10.6h), although their use remains largely at clinical trial stages.
Evidencing overall good tolerability and improvement in global health, the challenge of the current era relates to the availability of these therapies, and the critical need to compare and establish them against the approved standards of care across each point of the clinical pathways.
Artificial intelligence and big data in molecular imaging and therapy
The complexity and sheer volume of data generated by molecular imaging technologies like LAFOV PET-CT require advanced computational tools for data integration and interpretation. Machine learning and Artificial intelligence (AI) has emerged as a key enabler in nuclear medicine, with applications spanning image reconstruction and lesion detection.
AI algorithms are being developed to facilitate the use of more automated quantitative analysis of PET-CT and SPECT-CT images, improving the speed and accuracy of diagnosis. For example, AI can assist in quantifying tumour burden, assessing treatment response, and even predicting patient outcomes based on imaging data. Deep learning-based image processing software in the latest generation of PET-CT scanners has recently been reported to increase small, low-contrast lesion detectability (compared to conventional Time-Of-Flight (ToF) reconstruction commonly used in PET data for the last 10 years), thus potentially starting treatment and monitoring early on the disease process. Additionally, AI-driven segmentation tools facilitate the extraction of quantitative biomarkers (radiomics), which are essential for truly personalised treatment planning by maximising the radiation dose to tumours (and therapeutic efficacy) while minimising toxicity and keeping organs at risk within tolerance limits (13).
Application of deep learning-based techniques on non-attenuation corrected PET data has been used to generate attenuation correction maps (μ-maps), enabling CT-free attenuation and scatter correction in LAFOV PET scanners which may prove particularly beneficial for dose reduction in repeated scans or indeed in imaging of pregnant women and of children (22).
The large datasets generated by LAFOV PET-CT and other molecular imaging modalities provide a wealth of information that can be mined for predictive insights. By integrating patient imaging data with other clinical and molecular data, researchers can develop predictive models for disease progression, treatment response, and survival outcomes.
Conclusion
The field of molecular imaging and nuclear medicine is experiencing fast, transformative change with theranostics driving an “industrial revolution” in the field. These changes are being felt through technological enhancements of scanners, new imaging paradigms such as LAFOV PET and with newly developed imaging and therapeutic radiopharmaceuticals.
Each of these radiopharmaceuticals represents a significant advancement in nuclear medicine, improving diagnostic precision and enabling more targeted therapeutic options across various cancers and diseases. These agents illustrate the expanding toolkit in molecular imaging and therapy, providing clinicians with increasing flexibility to select the most appropriate radiopharmaceutical for individual patients and specific disease contexts. Nevertheless, it is crucial to forecast and invest in the necessary resources (including workforce numbers and continued training) and infrastructure to optimise the impact and ensure accessibility to all those who can benefit from them.
References
- Kiani M, Jokar S, Hassanzadeh L, Behnammanesh H, Bavi O, Beiki D, Assadi M. Recent Clinical Implications of FAPI: Imaging and Therapy. Clin Nucl Med. 2024 Nov 1;49(11):e538-e556. doi: 10.1097/RLU.0000000000005348. Epub 2024 Jul 17. PMID: 39025634.
- Mori Y, Dendl K, Cardinale J, Kratochwil C, Giesel FL, Haberkorn U. FAPI PET: Fibroblast Activation Protein Inhibitor Use in Oncologic and Nononcologic Disease. Radiology. 2023 Feb;306(2):e220749. doi: 10.1148/radiol.220749. Epub 2023 Jan 3. PMID: 36594838.
- Sidrak MMA, De Feo MS, Corica F, Gorica J, Conte M, Filippi L, Schillaci O, De Vincentis G, Frantellizzi V. Fibroblast Activation Protein Inhibitor (FAPI)-Based Theranostics-Where We Are at and Where We Are Heading: A Systematic Review. Int J Mol Sci. 2023 Feb 15;24(4):3863. doi: 10.3390/ijms24043863. PMID: 36835275; PMCID: PMC9965519.
- Martin M, Ballal S, Yadav MP, Bal C, Van Rymenant Y, De Loose J, Verhulst E, De Meester I, Van Der Veken P, Roesch F. Novel Generation of FAP Inhibitor-Based Homodimers for Improved Application in Radiotheranostics. Cancers (Basel). 2023 Mar 21;15(6):1889. doi: 10.3390/cancers15061889. PMID: 36980775; PMCID: PMC10047490.
- Zeven K, Lauwers Y, De Mey L, Debacker JM, De Pauw T, De Groof TWM, Devoogdt N. Advancements in nuclear imaging using radiolabeled nanobody tracers to support cancer immunotherapy. Immunother Adv. 2024 Aug 26;4(1):ltae006. doi: 10.1093/immadv/ltae006. PMID: 39281708; PMCID: PMC11402390.
- Glaudemans AWJM, Lammertsma AA, Cherry SR, Erba PA, Rominger A, Dierckx RAJO, Boellaard R, Hammers A, Hicks RJ, Slart RHJA. A cutting-edge technology for the future of nuclear medicine. Eur J Nucl Med Mol Imaging. 2024 Dec 11. doi: 10.1007/s00259-024-07028-7. Epub ahead of print. PMID: 39661126.
- Cook GJR, Alberts IL, Wagner T, Fischer BM, Nazir MS, Lilburn D. The impact of long axial field of view (LAFOV) PET on oncologic imaging. Eur J Radiol. 2024 Dec 4;183:111873. doi: 10.1016/j.ejrad.2024.111873. Epub ahead of print. PMID: 39647272.
- Borgwardt L, Brok J, Andersen KF, Madsen J, Gillings N, Fosbøl MØ, Denholt CL, Petersen IN, Sørensen LS, Enevoldsen LH, Oturai PS, Johannesen HH, Højgaard L, Schulze C, Saxtoft E, Andersen F, Fischer BM. Performing [18F]MFBG Long-Axial-Field-of-View PET/CT Without Sedation or General Anesthesia for Imaging of Children with Neuroblastoma. J Nucl Med. 2024 Aug 1;65(8):1286-1292. doi: 10.2967/jnumed.123.267256. PMID: 38960713; PMCID: PMC11294065.
- Smith CLC, Yaqub M, Wellenberg RHH, Knip JJ, Boellaard R, Zwezerijnen GJC. Ultra-low foetal radiation exposure in 18F-FDG PET/CT imaging with a long axial field-of-view PET/CT system. EJNMMI Phys. 2024 May 24;11(1):45. doi: 10.1186/s40658-024-00648-w. PMID: 38789880; PMCID: PMC11126546.
- Zilli T, Achard V, Dal Pra A, Schmidt-Hegemann N, Jereczek-Fossa BA, Lancia A, Ingrosso G, Alongi F, Aluwini S, Arcangeli S, Blanchard P, Conde Moreno A, Couñago F, Créhange G, Dirix P, Gomez Iturriaga A, Guckenberger M, Pasquier D, Sargos P, Scorsetti M, Supiot S, Tree AC, Zapatero A, Le Guevelou J, Ost P, Belka C. Recommendations for radiation therapy in oligometastatic prostate cancer: An ESTRO-ACROP Delphi consensus. Radiother Oncol. 2022 Nov;176:199-207. doi: 10.1016/j.radonc.2022.10.005. Epub 2022 Oct 10. PMID: 36228761.
- Alam MR, Singh SB, Thapaliya S, Shrestha S, Deo S, Khanal K. A Review of 177Lutetium-PSMA and 225Actinium-PSMA as Emerging Theranostic Agents in Prostate Cancer. Cureus. 2022 Sep 20;14(9):e29369. doi: 10.7759/cureus.29369. PMID: 36284803; PMCID: PMC9584169.
- Baum RP, Novruzov E, Zhao T, Greifenstein L, Jakobsson V, Perrone E, Mishra A, Eismant A, Ghai K, Klein O, Jaeschke B, Benz-Zils D, Cardinale J, Mori Y, Giesel FL, Zhang J. Radiomolecular Theranostics With Fibroblast-Activation-Protein Inhibitors and Peptides. Semin Nucl Med. 2024 Jul;54(4):537-556. doi: 10.1053/j.semnuclmed.2024.05.010. Epub 2024 Jul 16. PMID: 39019653.
- Belge Bilgin G, Bilgin C, Burkett BJ, Orme JJ, Childs DS, Thorpe MP, Halfdanarson TR, Johnson GB, Kendi AT, Sartor O. Theranostics and artificial intelligence: new frontiers in personalized medicine. Theranostics. 2024 Mar 25;14(6):2367-2378. doi: 10.7150/thno.94788. PMID: 38646652; PMCID: PMC11024845.
- Kratochwil C, Fendler WP, Eiber M, Hofman MS, Emmett L, Calais J, Osborne JR, Iravani A, Koo P, Lindenberg L, Baum RP, Bozkurt MF, Delgado Bolton RC, Ezziddin S, Forrer F, Hicks RJ, Hope TA, Kabasakal L, Konijnenberg M, Kopka K, Lassmann M, Mottaghy FM, Oyen WJG, Rahbar K, Schoder H, Virgolini I, Bodei L, Fanti S, Haberkorn U, Hermann K. Joint EANM/SNMMI procedure guideline for the use of 177Lu-labeled PSMA-targeted radioligand-therapy (177Lu-PSMA-RLT). Eur J Nucl Med Mol Imaging. 2023 Jul;50(9):2830-2845. doi: 10.1007/s00259-023-06255-8. Epub 2023 May 29. PMID: 37246997; PMCID: PMC10317889.
- Meyer C, Stuparu A, Lueckerath K, Calais J, Czernin J, Slavik R, Dahlbom M. Tandem Isotope Therapy with 225Ac- and 177Lu-PSMA-617 in a Murine Model of Prostate Cancer. J Nucl Med. 2023 Nov;64(11):1772-1778. doi: 10.2967/jnumed.123.265433. Epub 2023 Oct 5. PMID: 37797974; PMCID: PMC10626377.
- De Vincentis G, Gerritsen W, Gschwend JE, Hacker M, Lewington V, O'Sullivan JM, Oya M, Pacilio M, Parker C, Shore N, Sartor O; Targeted Alpha Therapy Prostate Working Group. Advances in targeted alpha therapy for prostate cancer. Ann Oncol. 2019 Nov 1;30(11):1728-1739. doi: 10.1093/annonc/mdz270. PMID: 31418764; PMCID: PMC6927314.
- Ma J, Li L, Liao T, Gong W, Zhang C. Efficacy and Safety of 225Ac-PSMA-617-Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2022 Feb 3;12:796657. doi: 10.3389/fonc.2022.796657. PMID: 35186737; PMCID: PMC8852230.
- Santo G, Di Santo G, Virgolini I. Peptide Receptor Radionuclide Therapy of Neuroendocrine Tumors: Agonist, Antagonist and Alternatives. Semin Nucl Med. 2024 Jul;54(4):557-569. doi: 10.1053/j.semnuclmed.2024.02.002. Epub 2024 Mar 15. PMID: 38490913.
- Gracheva N, Müller C, Talip Z, Heinitz S, Köster U, Zeevaart JR, Vögele A, Schibli R, van der Meulen NP. Production and characterization of no-carrier-added 161Tb as an alternative to the clinically-applied 177Lu for radionuclide therapy. EJNMMI Radiopharm Chem. 2019 Jul 10;4(1):12. doi: 10.1186/s41181-019-0063-6. PMID: 31659528; PMCID: PMC6620226.
- Krasnovskaya OO, Abramchuck D, Erofeev A, Gorelkin P, Kuznetsov A, Shemukhin A, Beloglazkina EK. Recent Advances in 64Cu/67Cu-Based Radiopharmaceuticals. Int J Mol Sci. 2023 May 23;24(11):9154. doi: 10.3390/ijms24119154. PMID: 37298101; PMCID: PMC10288943.
- Capriotti G, Piccardo A, Giovannelli E, Signore A. Targeting Copper in Cancer Imaging and Therapy: A New Theragnostic Agent. J Clin Med. 2022 Dec 28;12(1):223. doi: 10.3390/jcm12010223. PMID: 36615024; PMCID: PMC9821557.
- Sari H, Teimoorisichani M, Mingels C, Alberts I, Panin V, Bharkhada D, Xue S, Prenosil G, Shi K, Conti M, Rominger A. Quantitative evaluation of a deep learning-based framework to generate whole-body attenuation maps using LSO background radiation in long axial FOV PET scanners. Eur J Nucl Med Mol Imaging. 2022 Nov;49(13):4490-4502. doi: 10.1007/s00259-022-05909-3. Epub 2022 Jul 19. PMID: 35852557; PMCID: PMC9606046.
Image credit: Getty Images
Read more