By sonographers, for sonographers: innovation in fetal imaging
For the past 10 years Dr Jackie Matthew has been working on researching and developing an AI fetal ultrasound device. Synergy spoke to her about the process of creating a state of the art piece of healthcare technology
By sonographers, for sonographers: innovation in fetal imaging
For the past 10 years Dr Jackie Matthew has been working on researching and developing an AI fetal ultrasound device. Synergy spoke to her about the process of creating a state of the art piece of healthcare technology
By Will Phillips, staff writer
By Will Phillips, staff writer
By Will Phillips, staff writer
By Will Phillips, staff writer
“What is really unique about this,” says Dr Jackie Matthew, sonographer and co-founder of AI ultrasound tool firm Fraiya, “is how involved sonographers have been along the way.”
Jackie, a clinical senior research fellow in the Department of Early Life Imaging at King’s College London, has spent the past decade examining how AI can help – not just in terms of large-scale, cost and time-saving efficiencies, but in the day to day of hospital work.
Following a 10-year multi-disciplinary study using advanced engineering techniques, robotics and computer image analysis to develop AI applications for improving ultrasound, Jackie found herself at the forefront of a university spin-out focused on the design and development of an AI-enabled ultrasound medical device, which will support sonographers performing the 20-week anomaly scan, given to every pregnant woman. Based on early trial results, Jackie and her team founded Fraiya.
Sonography, like many diagnostic fields in the UK, is struggling with a lack of resources, a lack of training capacity and a fundamental lack of people – which makes it all the more impressive that so many came together to work on Fraiya’s AI ultrasound medical device and service.
Though the journey hasn’t been easy, Jackie says she has learned a lot along the way. That learning has continued throughout the start-up journey, on the NHS Clinical Entrepreneur Fellowship programme and as part of multiple innovation accelerator initiatives.
Synergy caught up with her to ask about the latest developments and what advice she would give to those looking to follow in her footsteps.

How it works
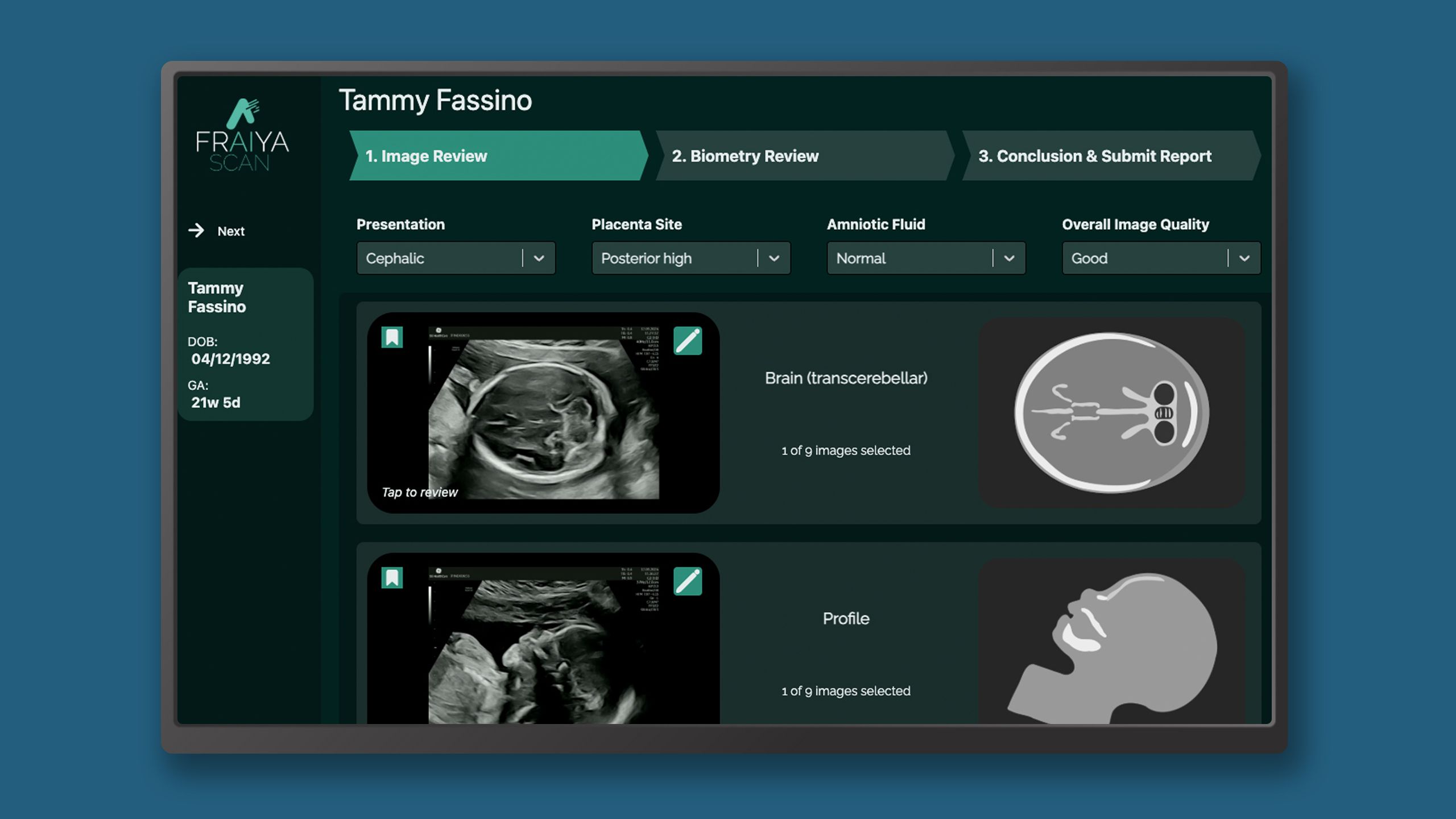
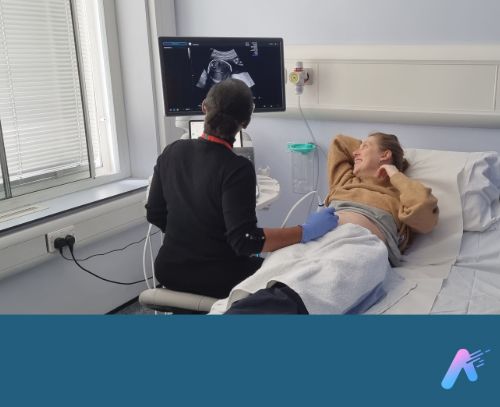
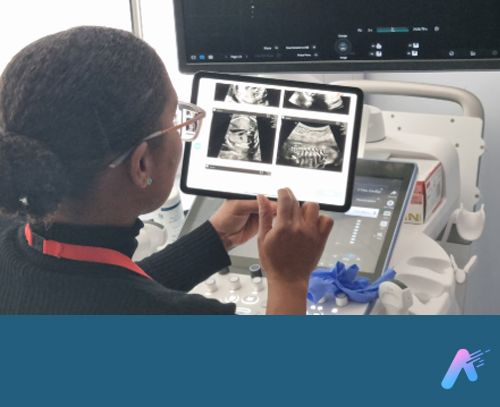
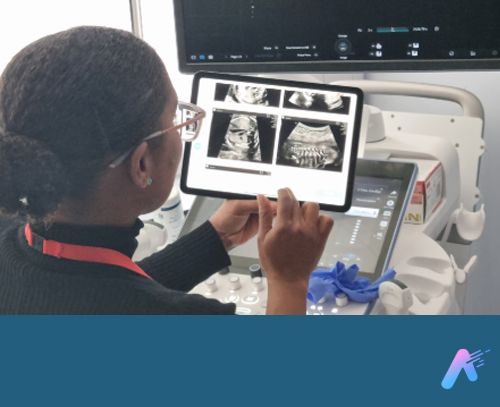
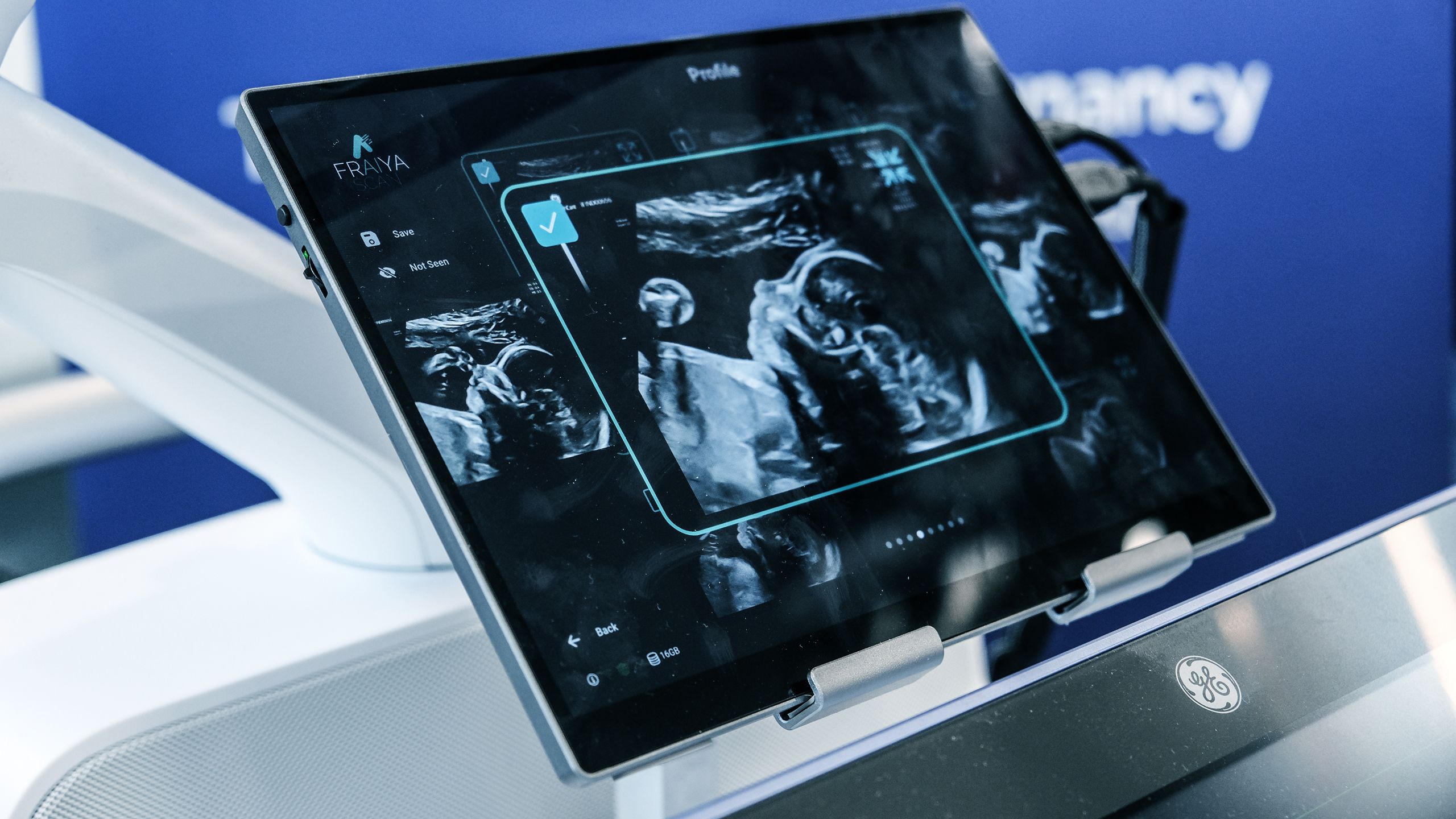
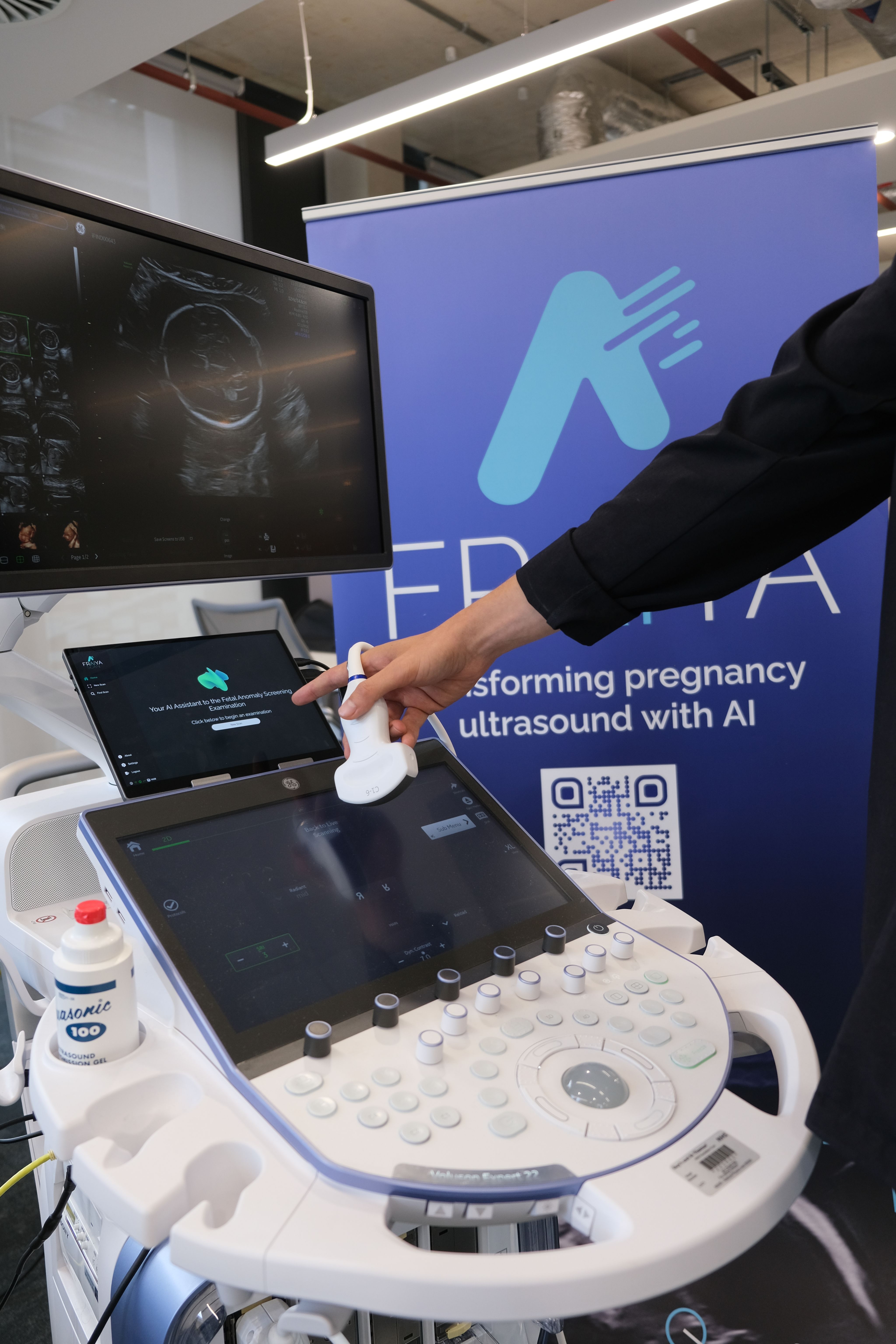
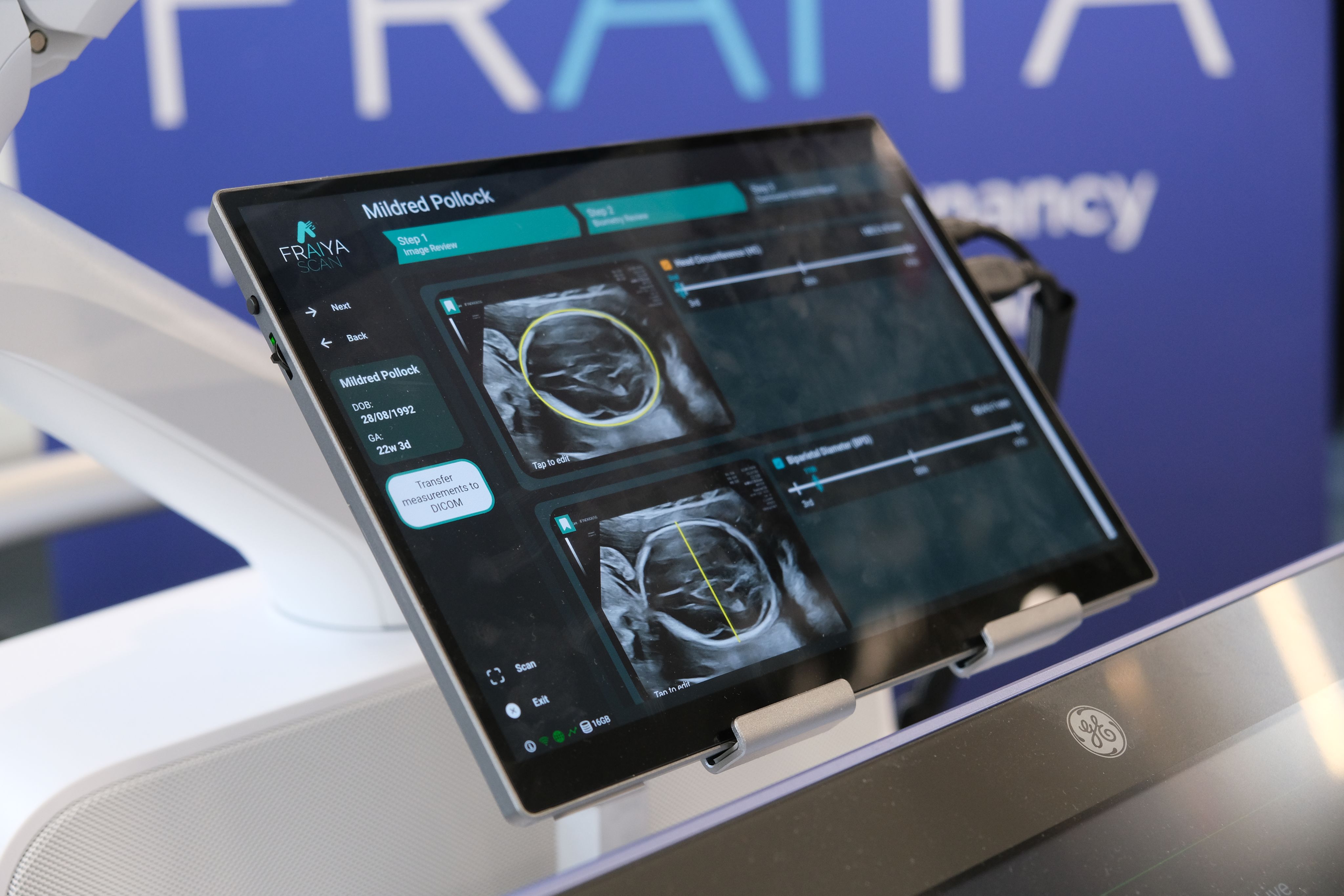
Fraiya’s suite of AI-enabled tools seeks to reduce examination times, improve screening and diagnosis, and work seamlessly across scanning machines. A tablet accessory connected to the ultrasound machine, using software called FraiyaScan, enables sonographers to receive real-time AI assistance for mid-trimester anomaly scans. Every scan can then be streamed asynchronously to remote expert reviewers, who can perform an AI-assisted peer review of each examination using their internal tool, FraiyaDetect.
As the software runs on a tablet, it can connect to any ultrasound machine, and automatically takes images and documents the procedure as part of the exam itself, including automatically taking measurements.
Recently, the results of a trial investigating its potential were published in The New England Journal of Medicine AI, and can be found online here. The study was a randomised, controlled trial using the device, and it discovered:
- AI-assisted scans were shorter in duration than standard scans (median 11.4 minutes vs 19.7 minutes).
- AI-assisted scan sensitivity and specificity for an enriched sample of congenital heart defects were 88.9 per cent and 98.0 per cent respectively, with the standard scan achieving 81.5 per cent and 92.2 per cent.
- Sonographer cognitive load was lower in the AI-assisted group, based on a NASA Task Load Index score of 35.2 versus 46.5.
- For all biometrics, the AI repeatability and reproducibility were superior to manual measurements as a result of reduced variability.
“It’s too early to say whether that will have an impact on actual detection rates in the real world. But of course, that would be the hope,” Jackie says.
The device will support sonographers to complete a comprehensive exam and take away some of the more repetitive tasks, so they can focus on assessing the baby.
Financial boost and sonographer feedback
The Fraiya team was awarded an Invention for Innovation Product Development Award from the National Institute for Health and Care Research (NIHR) in April, which supports collaborative research and development projects for medical devices. The award was a £1.7m grant to complete a multi-centre trial, with four sites across the UK, for a rigorous health economic evaluation and technology assessment.
This will help to understand the real-world impact of using a device like this in ultrasound services, as well as providing crucial evidence to support NHS procurement, required for broader adoption of new MedTech innovations.
Currently, it is difficult for screening ultrasound services to provide a comprehensive in-house second read for anomaly detection, given that very few static images are saved of the examination, and these fail to fully represent the real-time scan.
One of the endpoints of this trial is to assess changes in anomaly screening detection rates with the additional second reader service and peer-reviewed process. To this end, each scan is categorised by the AI into different body parts in the form of short clip for a rapid check, supported with AI diagnostic tools.
Any previously undetected anomalies, therefore, will be fed back to the department for management. “We’re really fortunate to have that close feedback loop with sonographers and fetal medicine specialists during the very early stages of development, and have considered how this may work in practice and what safeguards will need to be in place,” says Jackie.
“We’re trying to do something that could be really meaningful to and helpful for sonographers, and is rooted in actual clinical evidence – not AI as a commodity or a 'toy' that might be nice to go on a machine.”
Financial boost and sonographer feedback
The Fraiya team was awarded an Invention for Innovation Product Development Award from the National Institute for Health and Care Research (NIHR) in April, which supports collaborative research and development projects for medical devices. The award was a £1.7m grant to complete a multi-centre trial, with four sites across the UK, for a rigorous health economic evaluation and technology assessment.
This will help to understand the real-world impact of using a device like this in ultrasound services, as well as providing crucial evidence to support NHS procurement, required for broader adoption of new MedTech innovations.
Currently, it is difficult for screening ultrasound services to provide a comprehensive in-house second read for disease detection, given that very few static images are saved of the examination, and these fail to fully represent the real-time scan.
One of the endpoints of this trial is to assess changes in anomaly screening detection rates with the additional second reader service and peer-reviewed process. To this end, each scan is categorised by the AI into different body parts in the form of short clip for a rapid check, supported with AI diagnostic tools.
Any previously undetected anomalies, therefore, will be fed back to the department for management. “We’re really fortunate to have that close feedback loop with sonographers and fetal medicine specialists during the very early stages of development, and have considered how this may work in practice and what safeguards will need to be in place,” says Jackie.
“We’re trying to do something that could be really meaningful to and helpful for sonographers, and is rooted in actual clinical evidence – not AI as a commodity or a toy that might be nice to go on a machine.”
Patient information is automatically generated dummy patient data for demonstration purposes only
Patient information is automatically generated dummy patient data for demonstration purposes only
‘It’s about outcomes for the patient’
The original study closed in summer last year, with Fraiya as a company officially being ‘born’ at the end of 2024, having navigated NHS and university contractual and licensing agreements and early funding investments.
MedTech devices are subject to stringent regulatory processes, and software is no different. This is all in the interest of clinical safety, and Fraiya’s main product has recently achieved CE marking certification, giving the team much to celebrate. “Everything you do has to be evidence based,” Jackie explains. “We’re so excited to have achieved CE marking but this is also the start of an important phase… because it’s software, there will always be iterations in the product for improvements – that’s where the input from sonographers and the early adopters comes in. The sites we’re engaging with will be able to have early input into further development.”
The multi-centre trial will take place across a wide geographic spread, something Jackie pushed for. The team wants to understand what the impact of this device and the service in practice would be on health economics. “It’s not just about time saving, it’s asking what it means in terms of outcomes for the patient, outcomes for the health service,” she adds. “Did there end up being an increase in detection rates? Are there any consequences we didn’t anticipate that impact where this will be valuable to the healthcare system?”
While the trial is ongoing, each centre will also be conducting a process evaluation – a mixed methods study where the team will engage directly with patients who have been scanned with the AI device, as well as those who have gone through the normal pathway, to understand their take on the technology.
Finally, the sonographers themselves will be part of qualitative reviews to get their feedback on the experience of using the device to understand what the bottlenecks are for implementation and deployment – a particularly thorny area when it comes to AI systems, which need to be able to integrate and operate in concert with each other.
“We are a mission-driven company,” says Jackie. “We want to positively impact the ultrasound services. There’s a huge problem with sonographers suffering musculoskeletal repetitive strain injuries. We want to know if reducing scan time can help with that. Can having peer-review scans support them to feel able to deliver quality service? Does knowing there’s a safety net, a second pair of eyes reviewing scans, help with that?”
‘It’s about outcomes for the patient’
The original study closed in summer last year, with Fraiya as a company officially being ‘born’ at the end of 2024, having navigated NHS and university contractual and licensing agreements and early funding investments.
MedTech devices are subject to stringent regulatory processes, and software is no different. This is all in the interest of clinical safety, and Fraiya’s main product has recently achieved CE marking certification, giving the team much to celebrate. “Everything you do has to be evidence based,” Jackie explains. “We’re so excited to have achieved CE marking but this is also the start of an important phase… because it’s software, there will always be iterations in the product for improvements – that’s where the input from sonographers and the early adopters comes in. The sites we’re engaging with will be able to have early input into further development.”
The multi-centre trial will take place across a wide geographic spread, something Jackie pushed for. The team wants to understand what the impact of this device and the service in practice would be on health economics. “It’s not just about time saving, it’s asking what it means in terms of outcomes for the patient, outcomes for the health service,” she adds. “Did there end up being an increase in detection rates? Are there any consequences we didn’t anticipate that impact where this will be valuable to the healthcare system?”
While the trial is ongoing, each centre will also be conducting a process evaluation – a mixed methods study where the team will engage directly with patients who have been scanned with the AI device, as well as those who have gone through the normal pathway, to understand their take on the technology.
Finally, the sonographers themselves will be part of qualitative reviews to get their feedback on the experience of using the device to understand what the bottlenecks are for implementation and deployment – a particularly thorny area when it comes to AI systems, which need to be able to integrate and operate in concert with each other.
“We are a mission-driven company,” says Jackie. “We want to positively impact the ultrasound services. There’s a huge problem with sonographers suffering musculoskeletal repetitive strain injuries. We want to know if reducing scan time can help with that. Can having peer-review scans support them to feel able to deliver quality service? Does knowing there’s a safety net, a second pair of eyes reviewing scans, help with that?”
Rapidly evolving tech
“We’ve had wonderful sonographers who have been involved at different points in time,” says Jackie. These include Emily Skelton, a sonographer on the Ultrasound Advisory Group at the SoR, and Tara Fletcher, a sonographer in the research team, and many sonographers performing image analysis tasks.
“We worked very closely with engineers developing the technical side of the research – they were able to get our input into the realities of scanning in practice,” she continues. “Knowing what the service, patient and sonographer pain points are has been integral to developing what this product is now.”
Jackie still works as a clinical academic at King’s College, but part of her time is now devoted to working as one of the clinical leads, and chief medical officer, at Fraiya. “I work closely with the product and engineering team to develop the product and the offering,” she explains. “I work with key stakeholders, who might be from a fetal medicine background or might be sonographers who give us feedback in terms of usability and input into what they would expect a device like this to offer.”
Jackie is also part of the NHS Clinical Entrepreneur Programme, which supports healthcare staff to develop the commercial and innovation skills needed to bring new ideas into practice. “It’s been amazing to be part of that cohort,” she says. “The programme isn’t tied to any specific technology, but it’s a great way to build your confidence and capability if you’re exploring innovation or entrepreneurship.”
She’s particularly keen to see more radiographers get involved. “I’ve been promoting this programme for a number of years; if you’ve got ideas or even just curiosity about entrepreneurship, it’s a brilliant place to start. It’s not an easy path, and it’s very different to clinical practice, but it’s exciting. There are so many new roles and routes emerging and, with technology moving so fast, imaging and therapy staff are right at the centre of change.
“For me, research and innovation became a way to make a difference in my field. I’d encourage others, whether early in their career or more experienced, to explore what’s out there. The NIHR and other programmes are investing more than ever in supporting allied health professionals. If you’re interested and willing to step forward, there are real opportunities to grow and contribute.”
Find out more about Fraiya
Fraiya is a start-up from King’s College London and Guy’s and St Thomas’ NHS Foundation Trust, founded and led by a team of expert clinicians and engineers. Its goal is to create AI-powered tools for pregnancy ultrasound that change the way sonographers scan: improving medical diagnoses, empowering healthcare professionals and enhancing care for patients.
Find out more about Fraiya online here.
More about ultrasound and the SoR
The Ultrasound Advisory Group (UAG) provides the Society and College of Radiographers with advice regarding strategic direction for the profession and the appropriate support to deliver service improvements within the interprofessional environment of ultrasound. The remit of the group includes: promoting SoR | CoR’s policies, strategies and publications within the context of ultrasound to the benefit of services, practitioners and patients.
Find out more about the UAG here.
Read more